Cervical Cancer Test: New H.P.V. Test For Women Pushed Despite Medical Groups Rejection
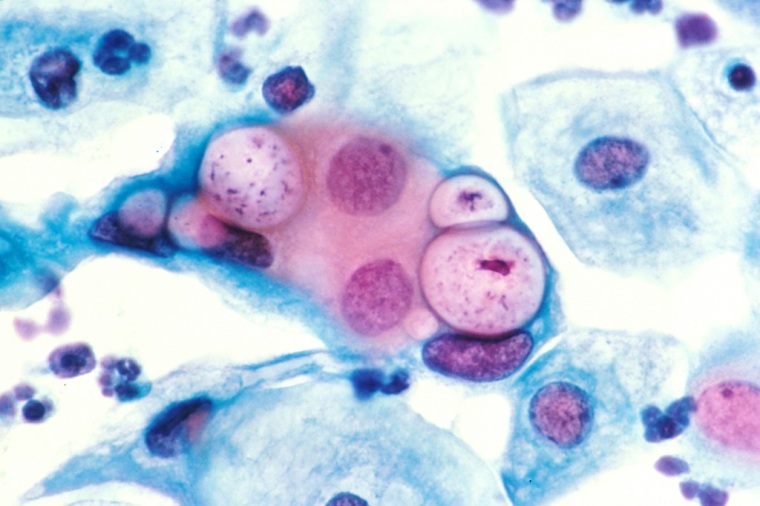
Two medical organizations have recommended getting a human papillomavirus (H.P.V.) test to assess women for cervical cancer before using Papanicolaou – or Pap – smear screening.
Although the new guidance, as published in Gynecologic Oncology, the Journal of Lower Genital Tract Disease and Obstetrics & Gynecology, is not accepted by all major medical groups, it gives some direction to doctors interested in using H.P.V. test for screening, according to lead author Dr. Warner Huh.
The H.P.V. test has been approved by the U.S. Food and Drug Administration (F.D.A.). But even more important than getting F.D.A. approval "is getting accurate information to providers so they can understand the approach," Huh was quoted by Reuters as saying.
The clinical guidance also seeks to give an overview of the potential advantages and disadvantages of using the strategy.
Over 12,000 women in the United States were diagnosed with cervical cancer while 4,092 in the country died from the disease, according to the latest statistics from the Center for Disease Control and Prevention (C.D.C.).
Nearly all cervical cancers, according to the C.D.C., are caused by H.P.V.
By race and ethnicity, black and Hispanic women have higher rates of H.P.V.-linked cervical cancer than white women.
Both the H.P.V. test and Pap test involve scraping cells from a woman's cervix and vagina for screening. The former checks for the presence of the virus while the latter looks for abnormal cells that may be pre-cancerous.
The new guidance suggests that women first be screened using an H.P.V. test at age 25. If the test is negative, they should not be screened for another three years.
If the H.P.V. test is positive for H.P.V. strains 16 and 18 – the ones most likely to lead to cancer – it should be followed by a biopsy of the cervix. If the women test positive for other strains of H.P.V., they should receive a Pap test.
For women younger than age 25, the new guidance says they should follow the current recommendation for Pap testing.
Last year, a study showed that H.P.V. test may be better than the Pap test at screening women for cervical cancer.
"Primary H.P.V. screening might be a viable alternative to Pap screening alone," said Julia Gage, the study's lead author from the National Institutes of Health's National Cancer Institute in Bethesda, Maryland.
The study used data from more than a million women between ages 30 and 64 and tested for the disease at Kaiser Permanente Northern California since 2003.
Researchers monitored women who had a negative Pap or H.P.V. test to check if they developed cervical cancer during the next three years. They also looked at how many women developed cervical cancer in the five years following co-testing.
Nearly 20 women out of 100,000 developed cervical cancer in the three years following a negative Pap test, compared to 11 women out of 100,000 who developed the cancer after receiving a negative H.P.V. test.
About 14 women out of 100,000 developed cervical cancer in the five years following negative co-tests, according to results published in the Journal of the National Cancer Institute.
 Christians don't have to affirm transgenderism, but they can’t express that view at work: tribunal
Christians don't have to affirm transgenderism, but they can’t express that view at work: tribunal Archaeology discovery: Medieval Christian prayer beads found on Holy Island
Archaeology discovery: Medieval Christian prayer beads found on Holy Island Presbyterian Church in America votes to leave National Association of Evangelicals
Presbyterian Church in America votes to leave National Association of Evangelicals Over 50 killed in 'vile and satanic' attack at Nigerian church on Pentecost Sunday
Over 50 killed in 'vile and satanic' attack at Nigerian church on Pentecost Sunday Ukrainian Orthodox Church severs ties with Moscow over Patriarch Kirill's support for Putin's war
Ukrainian Orthodox Church severs ties with Moscow over Patriarch Kirill's support for Putin's war Islamic State kills 20 Nigerian Christians as revenge for US airstrike
Islamic State kills 20 Nigerian Christians as revenge for US airstrike Man who served 33 years in prison for murder leads inmates to Christ
Man who served 33 years in prison for murder leads inmates to Christ


 Nigerian student beaten to death, body burned over ‘blasphemous’ WhatsApp message
Nigerian student beaten to death, body burned over ‘blasphemous’ WhatsApp message 'A new low': World reacts after Hong Kong arrests 90-year-old Cardinal Joseph Zen
'A new low': World reacts after Hong Kong arrests 90-year-old Cardinal Joseph Zen Iran sentences Christian man to 10 years in prison for hosting house church worship gathering
Iran sentences Christian man to 10 years in prison for hosting house church worship gathering French Guyana: Pastor shot dead, church set on fire after meeting delegation of Evangelicals
French Guyana: Pastor shot dead, church set on fire after meeting delegation of Evangelicals ‘Talking Jesus’ report finds only 6% of UK adults identify as practicing Christians
‘Talking Jesus’ report finds only 6% of UK adults identify as practicing Christians Mission Eurasia ministry center blown up in Ukraine, hundreds of Bibles destroyed: 'God will provide'
Mission Eurasia ministry center blown up in Ukraine, hundreds of Bibles destroyed: 'God will provide' Church holds service for first time after ISIS desecrated it 8 years ago
Church holds service for first time after ISIS desecrated it 8 years ago Burger King apologizes for 'offensive campaign' using Jesus' words at the Last Supper
Burger King apologizes for 'offensive campaign' using Jesus' words at the Last Supper Uganda: Muslims abduct teacher, burn him inside mosque for praying in Christ’s name
Uganda: Muslims abduct teacher, burn him inside mosque for praying in Christ’s name